update result found for ' atom '
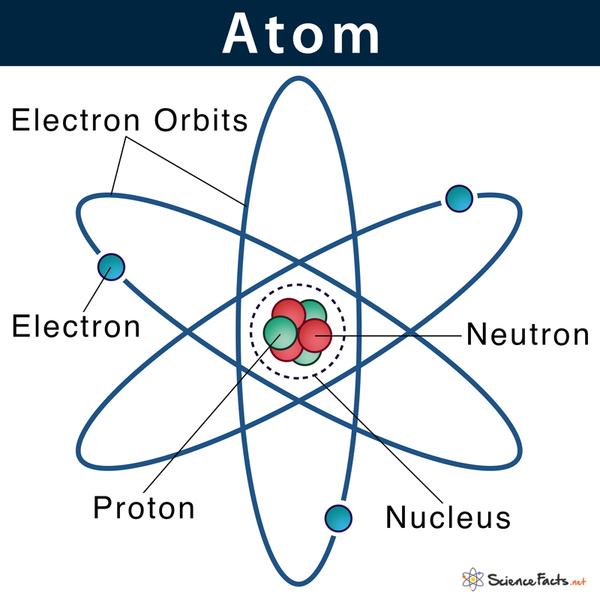
The structure of an atom consists of three main subatomic particles: protons, neutrons, and electrons. Here's a brief overview:Protons: Positively c
... 2024-04-23T05:47:02
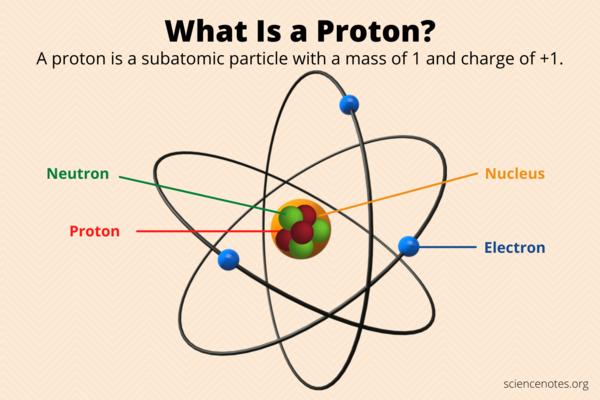
Protons are subatomic particles that are found within the nuclei of atoms. They are positively charged, with an electric charge of +1 elementary charg
... 2024-04-22T05:36:49
A covalent bond is a type of chemical bond that forms when atoms share one or more pairs of electrons between them. This sharing of electrons allows e
... 2024-04-02T05:07:57
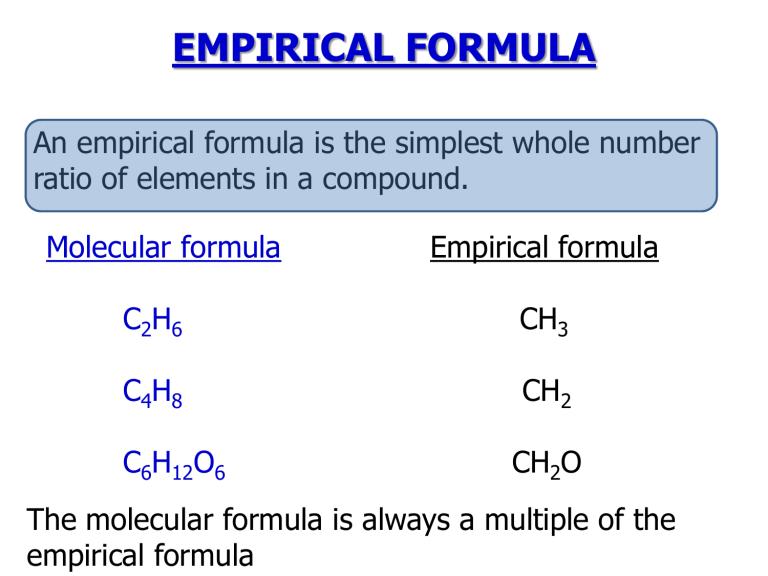
The empirical formula of a compound represents the simplest whole-number ratio of atoms of each element present in the compound. It gives the relative
... 2024-04-25T06:58:44
The empirical formula of a compound is the simplest whole number ratio of atoms of each element present in the compound. It gives the relative number
... 2024-04-11T05:28:44
The solid state of matter is one of the four fundamental states of matter, alongside liquid, gas, and plasma. Solids have a definite shape and volume,
... 2024-04-08T08:55:26
Hydrocarbons are organic compounds composed of only carbon and hydrogen atoms. They are fundamental to organic chemistry and play crucial roles in var
... 2024-04-25T07:02:02
The periodic table of elements is a tabular arrangement of all known chemical elements, organized based on their atomic number, electron configuration
... 2024-03-28T05:32:01
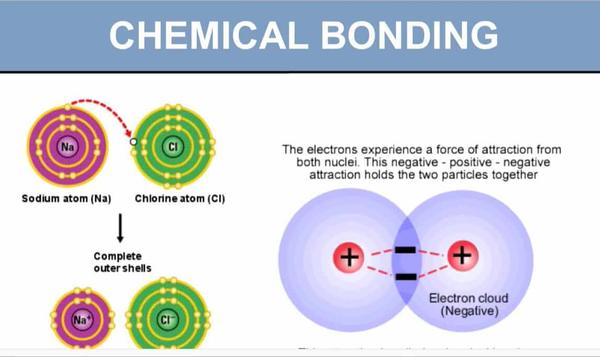
Chemical bonding refers to the attractive forces that hold atoms together in molecules and compounds. There are three main types of chemical bonds: co
... 2024-04-25T06:55:01