update result found for ' atoms '
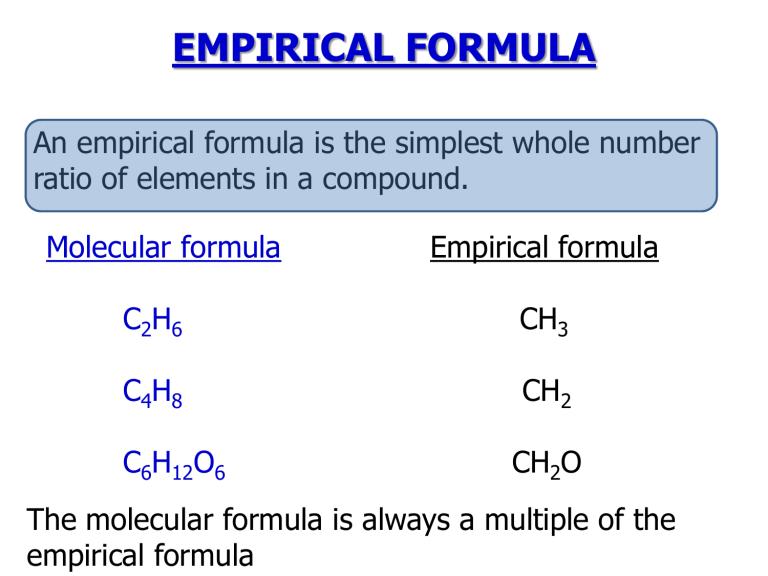
The empirical formula of a compound represents the simplest whole-number ratio of atoms of each element present in the compound. It gives the relative
... 2024-04-25T06:58:44
The empirical formula of a compound is the simplest whole number ratio of atoms of each element present in the compound. It gives the relative number
... 2024-04-11T05:28:44
A covalent bond is a type of chemical bond that forms when atoms share one or more pairs of electrons between them. This sharing of electrons allows e
... 2024-04-02T05:07:57
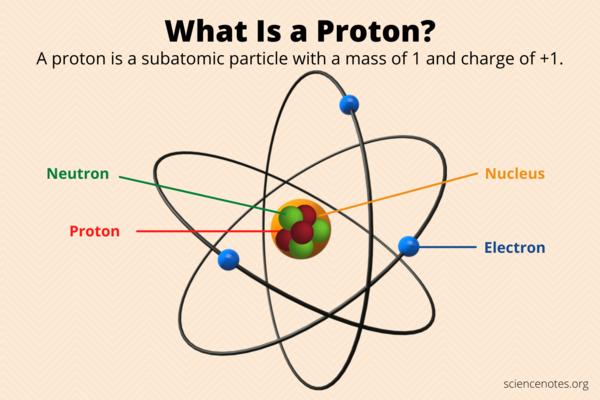
Protons are subatomic particles that are found within the nuclei of atoms. They are positively charged, with an electric charge of +1 elementary charg
... 2024-04-22T05:36:49
The solid state of matter is one of the four fundamental states of matter, alongside liquid, gas, and plasma. Solids have a definite shape and volume,
... 2024-04-08T08:55:26
Hydrocarbons are organic compounds composed of only carbon and hydrogen atoms. They are fundamental to organic chemistry and play crucial roles in var
... 2024-04-25T07:02:02
An electron is a subatomic particle with a negative electrical charge that orbits the nucleus of an atom. Electrons are one of the fundamental particl
... 2024-03-15T06:06:08
DNA, or deoxyribonucleic acid, is a molecule that contains the biological instructions for the development, functioning, growth, and reproduction of a
... 2024-03-14T06:10:06
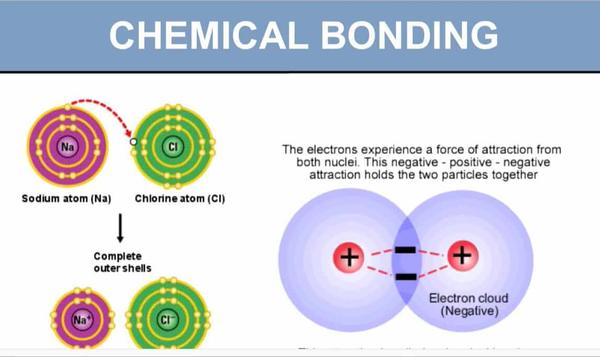
Chemical bonding refers to the attractive forces that hold atoms together in molecules and compounds. There are three main types of chemical bonds: co
... 2024-04-25T06:55:01